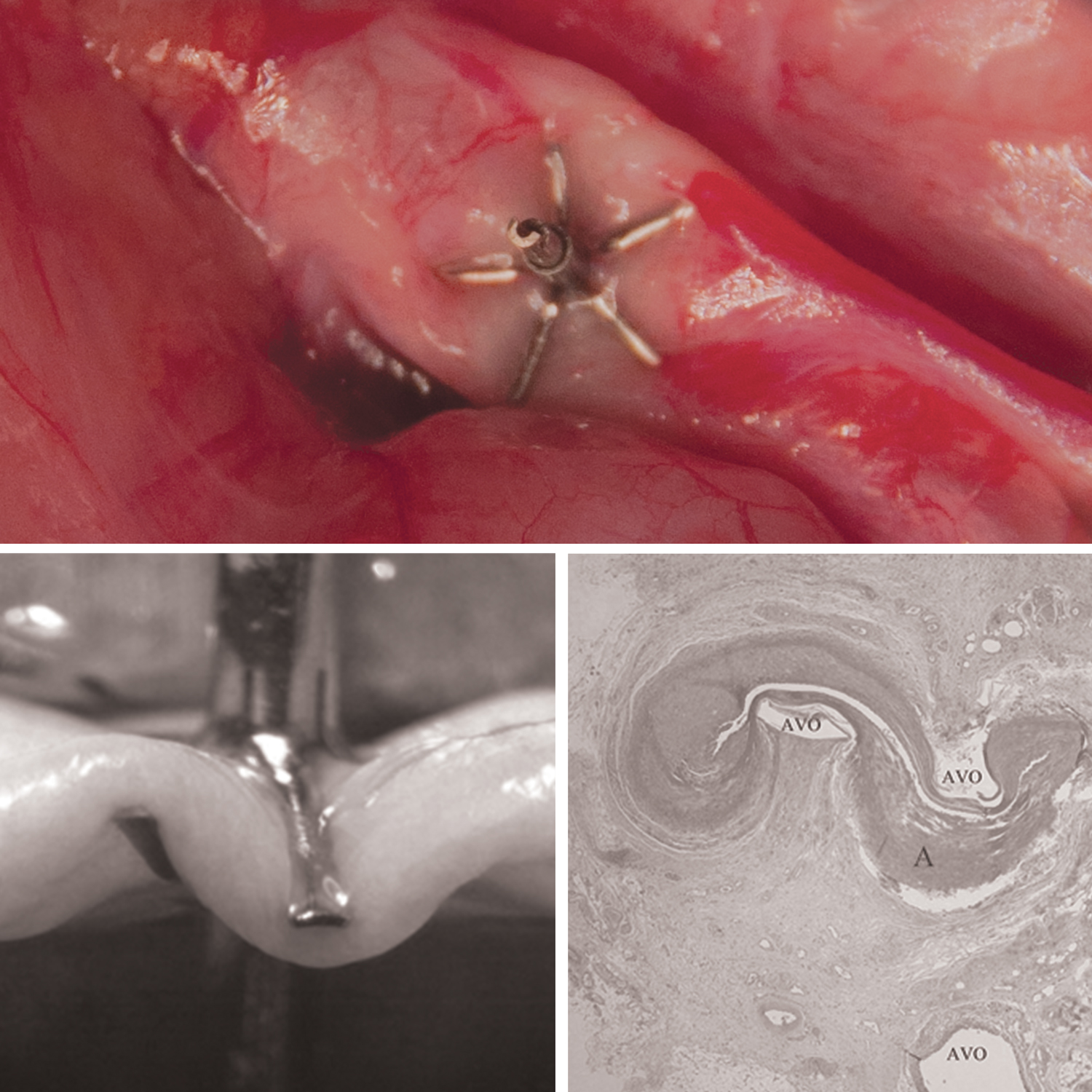
CE mark and FDA cleared SCureClamp™ with Interdigitation Occlusion Technology (iDOT™) is designed to easily and securely close vessels – veins, arteries, and tubular structures – during surgical and interventional procedures.
This unique mechanical surgical clamping technology is delivered directly through a fine, minimally invasively, 18 G needle for clamping of blood vessels, ducts, arteries, and veins. The minimally invasive needle delivery results in secure occlusion similar to that of a transfixion suture.
SCureClamp with iDOT’s anchoring feature secures the implant in place and eliminates slippage. Grasping allows physicians to occlude vessels of varying sizes, types and wall thickness.
Design Features:
- Permanent
- Non-Chemical
- Non-Thermal
- Non-Tumescent
- No Compression
- Ultrasound Guided
- Targeting 18 G Needle Delivery
FDA Indication for Use:
FDA Indication for use: The Amsel Occluder Device is intended for use in open general surgery procedures on tubular structures or vessels wherever a metal ligation clip is indicated and within the size range of 2.0mm to 7.00mm diameter.
CE Mark Indication for Use:
The Amsel Occluder device and its appliers are intended for use on tubular structures or vessels wherever a metal ligating clip is indicated. The tissue being ligated should be consistent with the size of the clip.
Watch to Learn More

Peer Reviewed Publications
Journal of Vascular Surgery
A preclinical animal study of a novel, simple, and secure percutaneous vessel occluder for the treatment of varicose veins
This study confirms that the SCureClamp with iDOT can be effectively delivered percutaneously under ultrasound guidance toocclude blood vessels in the porcine model and may be a useful, time-saving, and cost-effective adjunct to current primary methods of treating…
Journal of Surgical Endoscopy
A preclinical animal study of a novel, simple, and secure duct and vessel occluder for laparoscopic surgery
The SCureClamp with iDOT delivered laparoscopically provides an occlusion similar to a hand-sewn transfixion suture, is simple to use, and creates an occlusion which is not only more secure, but also as safe with respect to the health of the surrounding tissues, as that of the widely used hemoclip (Ligaclip®).
Journal of Surgical Research
A novel secure transfixing blood vessel occluder: comparison with the hemoclip in the porcine model
The SCureClamp with iDOT is simple to deploy and securely maintains occlusion by transfixing the targeted vessel, unlike the widely used, nontransfixing Ligaclip, that has a tendency to dislodge. As such, the SCureClamp with iDOT opens up numerous treatment opportunities in…
Specialties
VASCULAR
Current treatments for venous disease do not typically provide a permanent solution. Without effective occlusion, physicians are seeing ulcer recurrence, rising costs, and severe patient morbidity.
Perforator treatments such as lasers, RF, glue, and sclerotheray average a 75-80% efficacy rate.
“The veins in your body play an important role in circulation, carrying blood from your various parts of your body back to your heart. Yet, as people age, problems can develop in the veins and cause a variety of complications. In fact, one in three Americans over the age of 45 has some kind of vein disease.
Early symptoms may seem minor. However, they can become more serious – and even life-threatening – if they are not treated. Which is why it is important to be aware of symptoms and seek medical advice at the earliest sign of a problem.
”
Source: veinforum.org
87% of military fatalities occur in the prehospital environment
TRAUMA: MILITARY
The temporary clamping device is targeted at reducing front-line deaths associated with non-compressible and junctional hemorrhage, which currently accounts for a staggering 60% of fatalities in military trauma cases. Current data shows that 87% of military fatalities occur in the prehospital environment, proving the need for an accessible, portable, minimally invasive, and effective tool to aid in the urgent care of those wounded.
Currently Under Development:
SURGICAL APPLICATIONS
There are numerous general surgical applications for the SCureClamp with iDOT, including bariatric, cardiac and oncology.
- Vein Physicians
- General Surgeons
- Vascular Surgeons
- Cardiovascular Surgeons
- Interventional Radiologists
- Military Medical Personnel/Emergency Medical Technicians

From the Field
“The SCureClamp possesses the ability to transfix and ligate a selected vessel, advancing our technique beyond the current limitations of standard ligation clips.”
“The SCureClamp represents a fresh approach to facilitating accurate, complete and secure vessel closure.”